
Chemistry, 02.03.2020 21:03 liljobe8973
C6H5NH3Cl is a chloride salt with an acidic cation. If 46.3 g of C6H5NH3Cl is dissolved in water to make 150 mL of solution, what is the initial molarity of the cation

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
How air particles exert a pressure on the inside of the balloon
Answers: 1

Chemistry, 21.06.2019 23:00
At room temperature what happens to the average kinetic energy of the molecules of a solid, liquid, and a gas
Answers: 2

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1

Chemistry, 22.06.2019 19:50
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1
You know the right answer?
C6H5NH3Cl is a chloride salt with an acidic cation. If 46.3 g of C6H5NH3Cl is dissolved in water to...
Questions

Mathematics, 04.11.2020 23:30




Mathematics, 04.11.2020 23:30

Mathematics, 04.11.2020 23:30


Mathematics, 04.11.2020 23:30


Mathematics, 04.11.2020 23:30


Mathematics, 04.11.2020 23:30

Biology, 04.11.2020 23:30

History, 04.11.2020 23:30





Mathematics, 04.11.2020 23:30

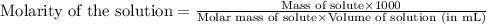
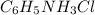 = 46.3 g
= 46.3 g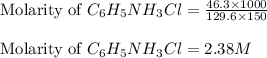
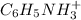 cation and 1 mole of
cation and 1 mole of 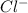 anion
anion

