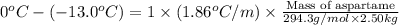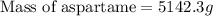Chemistry, 02.03.2020 21:28 crispingolfer1864
At − 13.0 ∘ C −13.0 ∘C , a common temperature for household freezers, what is the maximum mass of aspartame (C14H18N2O5) you can add to 2.50 kg 2.50 kg of pure water and still have the solution freeze? Assume that aspartame is a molecular solid and does not ionize when it dissolves in water.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
In pea plants, the allele for tallness (t) is dominant to the allele for shortness (t). in the cross between a tall pea plant and a short pea plant shown below, what is the probability that the resulting offspring will be tall? whats the percent
Answers: 1

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1

Chemistry, 22.06.2019 21:30
Harry lives in a city, and he has a lung condition known as asthma. on certain days, harry has to stay inside because pollutants in the air make it difficult for him to breathe. which of these pollution sources are nonpoint sources that might bother harry if he goes outside? choose the two that apply.
Answers: 3

Chemistry, 22.06.2019 22:30
What must be in balance for temperatures to remain constant?
Answers: 1
You know the right answer?
At − 13.0 ∘ C −13.0 ∘C , a common temperature for household freezers, what is the maximum mass of as...
Questions


Mathematics, 13.09.2021 06:30

English, 13.09.2021 06:30





Mathematics, 13.09.2021 06:30

Social Studies, 13.09.2021 06:30

Mathematics, 13.09.2021 06:30

Mathematics, 13.09.2021 06:30

Social Studies, 13.09.2021 06:30





Mathematics, 13.09.2021 06:30

English, 13.09.2021 06:30

Mathematics, 13.09.2021 06:30

Computers and Technology, 13.09.2021 06:30

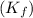 for water =
for water = 
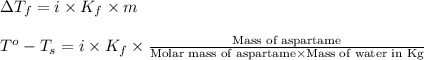
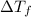 = change in freezing point
= change in freezing point = freezing point of solution =
= freezing point of solution = 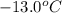
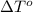 = freezing point of water =
= freezing point of water = 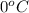
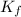 = freezing point constant for water =
= freezing point constant for water =