
Chemistry, 02.03.2020 21:50 youngchapo813p8d9u1
If the pH of a somewhat dilute (i. e. ~0.08 to 0.10 M) aqueous solution of HCl is 1.14, then to a good approximation, what is the concentration of hydrogen ions in this solution? Enter your answer to the nearest hundredth.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2

Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1

Chemistry, 23.06.2019 05:30
According to thomson, the atom is a positively charged cloud with electrons scattered throughout. what would the alpha particles do when they hit the foil if thomson were correct
Answers: 1

Chemistry, 23.06.2019 09:10
A155.0 9 piece of copper at 182 °c is dropped into 2500 g of water at 23.9 °c. (the specific heat of copper is 0.385 1/9°c.) calculate the final temperature of the mixture. (assume no heat loss to the surroundings.)
Answers: 2
You know the right answer?
If the pH of a somewhat dilute (i. e. ~0.08 to 0.10 M) aqueous solution of HCl is 1.14, then to a go...
Questions


Social Studies, 29.11.2020 14:50

Mathematics, 29.11.2020 14:50


English, 29.11.2020 14:50















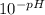 =
= 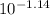 = 0.0724 M= 0.07 M
= 0.0724 M= 0.07 M

