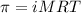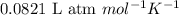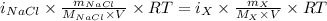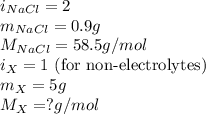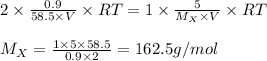Chemistry, 02.03.2020 23:07 bryanmcmillianjr
The osmotic pressure of a 0.9 weight percent solution of sodium chloride (NaCl) is equal to the osmotic pressure of a 5 weight percent solution of non-dissociating molecule X. What is the molecular weight of molecule X

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:50
How will the emission of an alpha particle affect the atomic number of an atom
Answers: 3

Chemistry, 22.06.2019 06:00
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3


Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3
You know the right answer?
The osmotic pressure of a 0.9 weight percent solution of sodium chloride (NaCl) is equal to the osmo...
Questions


Computers and Technology, 14.05.2021 22:10


Mathematics, 14.05.2021 22:10

Physics, 14.05.2021 22:10

Biology, 14.05.2021 22:10


Computers and Technology, 14.05.2021 22:10

Mathematics, 14.05.2021 22:10




Mathematics, 14.05.2021 22:10

Mathematics, 14.05.2021 22:10



Mathematics, 14.05.2021 22:10

Social Studies, 14.05.2021 22:10

Mathematics, 14.05.2021 22:10

English, 14.05.2021 22:10