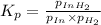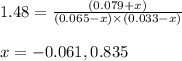Chemistry, 02.03.2020 23:05 shukriabdisabrie
Gaseous indium dihydride is formed from the elements at elevated temperature:
ln(g)+H2(g)?lnH2(g)Kp = 1.48 at 973 K
Partial pressures measured in a reaction vessel are:
PIn = 0.0650atm , PH2 = 0.0330atm , PInH2 = 0.0790atm
1. Calculate Qp
2. Determine the direction of reaction to attain equilibrium
3. Determine the equilibrium partial pressure of In
4. Determine the equilibrium partial pressure of H2
5. Determine the equilibrium partial pressure of InH2

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 04:30
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1
You know the right answer?
Gaseous indium dihydride is formed from the elements at elevated temperature:
ln(g)+H2(g...
ln(g)+H2(g...
Questions

English, 07.09.2020 15:01

Business, 07.09.2020 15:01

English, 07.09.2020 15:01

Spanish, 07.09.2020 15:01

Mathematics, 07.09.2020 15:01

Social Studies, 07.09.2020 15:01


Mathematics, 07.09.2020 15:01

Geography, 07.09.2020 15:01

Geography, 07.09.2020 15:01



Mathematics, 07.09.2020 15:01

English, 07.09.2020 15:01

Mathematics, 07.09.2020 15:01

Chemistry, 07.09.2020 15:01

Mathematics, 07.09.2020 15:01


Mathematics, 07.09.2020 15:01

English, 07.09.2020 15:01

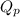 for above reaction is 36.83
for above reaction is 36.83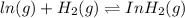
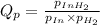
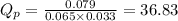
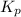 of the reaction = 1.48
of the reaction = 1.48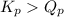 ; the reaction is product favored.When
; the reaction is product favored.When 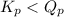 ; the reaction is reactant favored.When
; the reaction is reactant favored.When 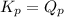 ; the reaction is in equilibrium.
; the reaction is in equilibrium.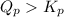 for the given reaction, the reaction is reactant favored.
for the given reaction, the reaction is reactant favored.