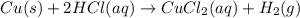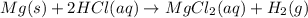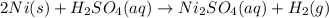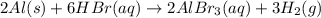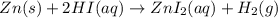Chemistry, 02.03.2020 23:21 raprocksbob
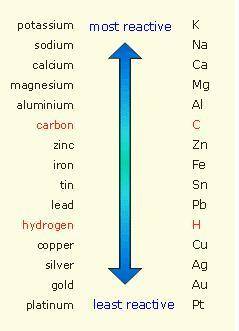
Which of the following reactions is not spontaneous? Cu (s) + 2HCl (aq) → CuCl2 (aq) + H2 (g) Mg (s) + 2HCl (aq) → MgCl2 (aq) + H2(g) 2Ni (s) + H2SO4 (aq) → Ni2SO4 (aq) + H2 (g) 2Al (s) + 6HBr (aq) → 2AlBr3 (aq) + 3H2 (g) Zn (s) + 2HI (aq) → ZnI2(aq) + H2 (g)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Will mark brainliest 26. which of these statements are true? (3 points) a. gases are compressible b. gases fill their containers completely c. the pressure of a gas is independent of the temperature d. gases have mass e. gases exert pressure f. the pressure of a gas is dependent on the volume g. gas pressure results from the collisions between gas particles h. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 16:40
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3
You know the right answer?
Which of the following reactions is not spontaneous? Cu (s) + 2HCl (aq) → CuCl2 (aq) + H2 (g) Mg (s)...
Questions










SAT, 09.02.2022 14:00

Mathematics, 09.02.2022 14:00




Mathematics, 09.02.2022 14:00

Chemistry, 09.02.2022 14:00

Mathematics, 09.02.2022 14:00