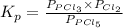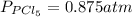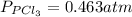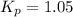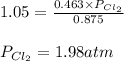Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1
You know the right answer?
The equilibrium constant for the decomposition of PCl5 at 250 celcius is 1.05. PCl5(g)-->PCl3(g)+...
Questions



Mathematics, 26.05.2020 20:59



Mathematics, 26.05.2020 20:59



Mathematics, 26.05.2020 20:59

Health, 26.05.2020 21:00


Arts, 26.05.2020 21:00


Mathematics, 26.05.2020 21:00



Medicine, 26.05.2020 21:00

Mathematics, 26.05.2020 21:00


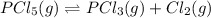
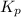 for above reaction follows:
for above reaction follows: