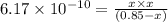Chemistry, 02.03.2020 23:29 ramirezzairap2u4lh
Use the "x is small" approximation to find the concentration of the products in the following reaction which initially contains only 0.85 M HCN. Decide whether using the approximation was valid or invalid. HCN(aq) ⇌ H+(aq) + CN−(aq), Kc= 6.17 x 10−10

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1


Chemistry, 23.06.2019 07:00
Determine the length of the object shown. 97.8 mm 97.80 mm 97 mm 98 mm
Answers: 1

Chemistry, 23.06.2019 07:30
In a laboratory determination of the atomic weight of tin, a sample of tin is weighed in a crucible. nitric acid is added, and the reaction proceeds to give a hydrated tin(iv)oxide plus no2and h2o. the hydrated tin(iv)oxide is then heated strongly and reacts as follows: sno2.xh2o(s)sno2(s)+ xh2o(g)the sno2is finally cooled and weighed in the crucible. explain the effect on the calculated atomic weight of tin that would result from each of the following experimental errors: (a)considerable spattering occurs when the nitric acid is added to the tin.(b)the hydrated tin(iv)oxide is not heated sufficiently to change it completely to tin oxide.
Answers: 2
You know the right answer?
Use the "x is small" approximation to find the concentration of the products in the following reacti...
Questions

Mathematics, 06.11.2020 17:10

Health, 06.11.2020 17:10






Mathematics, 06.11.2020 17:10


Mathematics, 06.11.2020 17:10

Chemistry, 06.11.2020 17:10

English, 06.11.2020 17:10








![[H^+]=0.0000229 M](/tpl/images/0531/0835/b0212.png)
![[CN^-]=0.0000229 M](/tpl/images/0531/0835/20a85.png)
![K_c=\frac{[H^+][CN^-]}{[HCN]}](/tpl/images/0531/0835/77800.png)