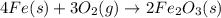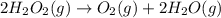Chemistry, 03.03.2020 00:02 snowprincess99447
For each of the reactions at constant pressure, determine whether the system does work on the surroundings, the surroundings does work on the system, or essentially no work is performed.
(a) 4Fe + 3O2 --> 2Fe2O3
(b) 2H2O2 --> O2 + 2H2O

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
As you move from right to left on the periodic table the atomic radius fill in the blank
Answers: 2

Chemistry, 21.06.2019 20:30
Hannah is writing a report on how albedo affects the global climate. she’s proofreading her passage for any factual errors. which sentence must hannah correct before submitting her report? earth receives energy from the sun. this energy drives many of the processes on earth, including its climate. some part of this energy is reflected by earth’s surface. we use the term albedo to describe the reflected energy. albedo of an object is the ratio of the reflected radiation to the total radiation reaching the object. a value of 0 means no energy is absorbed by the object, whereas a value of 1 means that all of the energy is absorbed. in this way, the albedo of an object can influence earth’s atmospheric temperature.
Answers: 1

Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2
You know the right answer?
For each of the reactions at constant pressure, determine whether the system does work on the surrou...
Questions

Mathematics, 28.10.2020 17:30


Social Studies, 28.10.2020 17:30



Social Studies, 28.10.2020 17:30


Social Studies, 28.10.2020 17:30


History, 28.10.2020 17:30


Mathematics, 28.10.2020 17:30

History, 28.10.2020 17:30


Mathematics, 28.10.2020 17:30


Social Studies, 28.10.2020 17:30

History, 28.10.2020 17:30


Mathematics, 28.10.2020 17:30