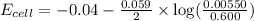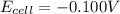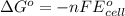Chemistry, 03.03.2020 00:06 cheyennebatz3609
Calculate the emf for the following reaction. Will the reaction occur spontaneously at 25°C, given that [Fe2+] = 0.600 M and [Cd2+] = 0.00550 M? Cd(s) + Fe2+(aq)→Cd2+(aq) + Fe(s) E o Cd2+/Cd = −0.40 V E o Fe2+/Fe = −0.44 V

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2


Chemistry, 23.06.2019 02:00
As light moves from one material into the next, which of the following affects how much the light waves will refract, or bend? angle at which the ray strikes the medium color of the material density of the material temperature of the light wave
Answers: 2

Chemistry, 23.06.2019 11:00
The image below shows a weather service map.. i’m not sure if is correct
Answers: 2
You know the right answer?
Calculate the emf for the following reaction. Will the reaction occur spontaneously at 25°C, given t...
Questions


Mathematics, 21.01.2021 03:10

Spanish, 21.01.2021 03:10

Mathematics, 21.01.2021 03:10


Mathematics, 21.01.2021 03:10

History, 21.01.2021 03:10

Biology, 21.01.2021 03:10

English, 21.01.2021 03:10

Mathematics, 21.01.2021 03:10

Chemistry, 21.01.2021 03:10

English, 21.01.2021 03:10



English, 21.01.2021 03:10



Mathematics, 21.01.2021 03:10

English, 21.01.2021 03:10

Mathematics, 21.01.2021 03:10

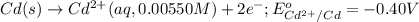
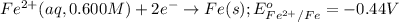
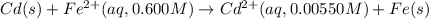
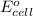 of the reaction, we use the equation:
of the reaction, we use the equation: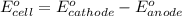
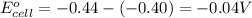
![E_{cell}=E^o_{cell}-\frac{0.059}{n}\log \frac{[Cd^{2+}]}{[Fe^{2+}]}](/tpl/images/0531/1947/ee65e.png)
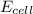 = electrode potential of the cell = ?
= electrode potential of the cell = ?![[Cd^{2+}]=0.00550M](/tpl/images/0531/1947/3e0ad.png)
![[Fe^{2+}]=0.600M](/tpl/images/0531/1947/5e50b.png)