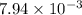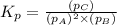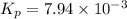Chemistry, 02.03.2020 23:58 jenellalexis94p5jp7i
The partial pressures of the gases when at equilibrium are found to be: A: 6.70 atm, B: 10.1 atm, C: 3.60 atm. Evaluate Kp for this reaction. 2A(g) B(g) --> C(g)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1


Chemistry, 23.06.2019 02:30
Ascientist wants to know how individual lions within a pride interact with each other in their own environment. to do this, the scientist sedates and tags all of the lions within a pride. then, he places several remotely-controlled video cameras near the lions' den and performs an observational field study. he collects continuous video footage over the span of one year, analyzes the video, and then forms conclusions based on his observations.
Answers: 2

Chemistry, 23.06.2019 03:40
Write the overall equation for the reaction occurring in lithium battery?
Answers: 3
You know the right answer?
The partial pressures of the gases when at equilibrium are found to be: A: 6.70 atm, B: 10.1 atm, C:...
Questions

Mathematics, 10.12.2020 20:20

Mathematics, 10.12.2020 20:20






Mathematics, 10.12.2020 20:20



Biology, 10.12.2020 20:20

Biology, 10.12.2020 20:20

Mathematics, 10.12.2020 20:20



English, 10.12.2020 20:20