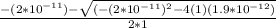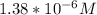Chemistry, 03.03.2020 01:01 ELGuapo6746
Calculate the pH at the equivalence point for the titration of 0.190 M 0.190 M methylamine ( CH 3 NH 2 ) (CH3NH2) with 0.190 M HCl . 0.190 M HCl. The K b Kb of methylamine is 5.0 × 10 − 4 .

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
The drawing represents the movement of particles in a substance. what changes of state can this substance undergo
Answers: 1

Chemistry, 21.06.2019 23:00
Which statement describes covalent bases? they have hydroxide ions. they produce hydrogen ions. they are often amines. they are named the same as ionic compounds.
Answers: 3

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2
You know the right answer?
Calculate the pH at the equivalence point for the titration of 0.190 M 0.190 M methylamine ( CH 3 NH...
Questions

Computers and Technology, 14.09.2019 05:30




Computers and Technology, 14.09.2019 05:30

Computers and Technology, 14.09.2019 05:30



Computers and Technology, 14.09.2019 05:30

Computers and Technology, 14.09.2019 05:30

Computers and Technology, 14.09.2019 05:30

Computers and Technology, 14.09.2019 05:30

Computers and Technology, 14.09.2019 05:30

Computers and Technology, 14.09.2019 05:30

Computers and Technology, 14.09.2019 05:30

Computers and Technology, 14.09.2019 05:30

Computers and Technology, 14.09.2019 05:30

Computers and Technology, 14.09.2019 05:30

Computers and Technology, 14.09.2019 05:30

Computers and Technology, 14.09.2019 05:30

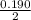
![K_a =\frac{[CH_3NH_2][H^+]}{[CH_3NH^+_3]}](/tpl/images/0531/3181/3871d.png)
![K_a = \frac{[x][x]}{[0.095-x]}](/tpl/images/0531/3181/0ca50.png)
![K_a = \frac{[x^2]}{[0.095-x]}](/tpl/images/0531/3181/85100.png) ------ equation (1)
------ equation (1)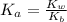
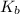 =
= 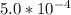 ; and
; and 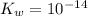 ;
;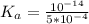
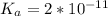
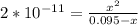
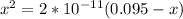
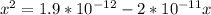

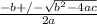 ; we have
; we have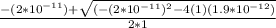 OR
OR