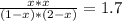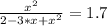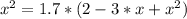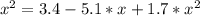Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which supports the idea that birds and butterflies both have wings but they do not have a common ancestor with wings? a. the wings are analogous structures that evolved differently and do not have a similar internal structure. b. the wings are homologous structures that evolved differently and do not have a similar internal structure. c. wings of birds are vestigial structures, but the wing structures of bats are not vestigial. d. wings of bats are vestigial structures, but the wing structures of birds are not vestigial
Answers: 1

Chemistry, 22.06.2019 01:00
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1

Chemistry, 22.06.2019 09:00
Identify the electromagnets with poles that are reversed from the electromagnet shown above
Answers: 3

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2
You know the right answer?
The reversible chemical reaction A+B⇌C+D has the following equilibrium constant: Kc=[C][D][A][B]=1.7...
Questions




Chemistry, 04.06.2020 23:01






Mathematics, 04.06.2020 23:01

Mathematics, 04.06.2020 23:01



English, 04.06.2020 23:01


Mathematics, 04.06.2020 23:01

Mathematics, 04.06.2020 23:01



Biology, 04.06.2020 23:01

![Kc=\frac{[C]^{c} *[D]^{d} }{[A]^{a} *[B]^{b} }](/tpl/images/0531/2647/eda24.png)
![Kc=\frac{[C]*[D]}{[A]*[B]}=1.7](/tpl/images/0531/2647/e4842.png)