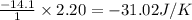Chemistry, 03.03.2020 01:25 KenzieD7876
H2(g) + F2(g)2HF(g) Using standard thermodynamic data at 298K, calculate the entropy change for the surroundings when 2.20 moles of H2(g) react at standard conditions. S°surroundings = J/K

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1

Chemistry, 22.06.2019 10:30
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2

Chemistry, 22.06.2019 22:00
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1

You know the right answer?
H2(g) + F2(g)2HF(g) Using standard thermodynamic data at 298K, calculate the entropy change for the...
Questions




Mathematics, 03.04.2020 06:35

Spanish, 03.04.2020 06:35



Mathematics, 03.04.2020 06:35



History, 03.04.2020 06:36

Advanced Placement (AP), 03.04.2020 06:36



Mathematics, 03.04.2020 06:37




Mathematics, 03.04.2020 06:37

Mathematics, 03.04.2020 06:37

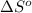 for the surrounding when given amount of hydrogen gas is reacted is -31.02 J/K
for the surrounding when given amount of hydrogen gas is reacted is -31.02 J/K![\Delta S^o_{rxn}=\sum [n\times \Delta S^o_{(product)}]-\sum [n\times \Delta S^o_{(reactant)}]](/tpl/images/0531/3518/52737.png)
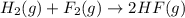
![\Delta S^o_{rxn}=[(2\times \Delta S^o_{(HF(g))})]-[(1\times \Delta S^o_{(H_2(g))})+(1\times \Delta S^o_{(F_2(g))})]](/tpl/images/0531/3518/0a21f.png)
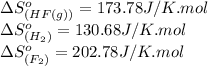
![\Delta S^o_{rxn}=[(2\times (173.78))]-[(1\times (130.68))+(1\times (202.78))]\\\\\Delta S^o_{rxn}=14.1J/K](/tpl/images/0531/3518/7b4e1.png)