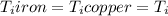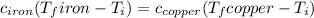Five-gram samples of copper and iron are at room temperature. both samples receive equal amounts of energy due to heat flow. the specific heat capacity of copper is 0.09 cal/g°c, and the specific heat capacity of iron is 0.11 cal/g°c. which of the following statements is true? the temperature of each sample will increase by the same amount. the temperature of each sample will decrease by the same amount. the copper will get hotter than the iron. the iron will get hotter than the copper.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
You are to give ampicillin with a recommended dose of 25mg/kg to a child with a mass of 29kg. if stock on hand is 250mg/capsule how many capsules should be given?
Answers: 1

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2

Chemistry, 23.06.2019 04:31
2ki + pb(no3)2 → 2kno3 + pbi2 determine how many moles of kno3 are created if 0.03 moles of ki are completely consumed.
Answers: 1
You know the right answer?
Five-gram samples of copper and iron are at room temperature. both samples receive equal amounts of...
Questions




English, 27.08.2020 18:01



Mathematics, 27.08.2020 18:01





Mathematics, 27.08.2020 18:01


Mathematics, 27.08.2020 18:01






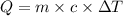
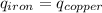
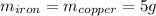 , also
, also