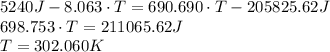A 62.5-g piece of gold at 650. K is dropped into 165 g of H2O (l) at 298 K in an insulated container at 1 bar pressure. Calculate the temperature of the system once equilibrium has been reached. Assume that CP, m for Au and H2O are constant at their values for 298 K throughout the temperature range of interest.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
The pressure in a fluid is affected by which characteristics of that fluid
Answers: 1


Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1

Chemistry, 22.06.2019 18:40
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1
You know the right answer?
A 62.5-g piece of gold at 650. K is dropped into 165 g of H2O (l) at 298 K in an insulated container...
Questions


Social Studies, 01.12.2020 22:50

History, 01.12.2020 22:50


Mathematics, 01.12.2020 22:50

Mathematics, 01.12.2020 22:50


Mathematics, 01.12.2020 22:50


Chemistry, 01.12.2020 22:50


Chemistry, 01.12.2020 22:50

Mathematics, 01.12.2020 22:50


Mathematics, 01.12.2020 22:50

History, 01.12.2020 22:50

History, 01.12.2020 22:50



Social Studies, 01.12.2020 22:50

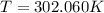
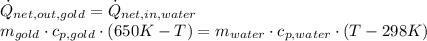
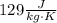 and
and 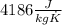 . Last expression is simplified by substituting known variables:
. Last expression is simplified by substituting known variables: