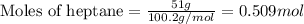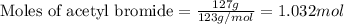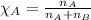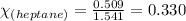Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1

Chemistry, 23.06.2019 05:30
Idont understand it 1.what is the boiling point of a solution of 675 grams of ethylene glycol (c2h6o2) in 2.50 liters of water?
Answers: 2

Chemistry, 23.06.2019 15:30
Which answer below correctly identifies the type of change and the explanation when magnesium comes into contact with hydrochloric acid
Answers: 1

Chemistry, 23.06.2019 18:00
What pair of body systems provide the raw materials that cells need for energy
Answers: 1
You know the right answer?
A solution is made by mixing of 51 g of heptane and of acetyl bromide . Calculate the mole fraction...
Questions




History, 04.02.2020 15:45

Geography, 04.02.2020 15:45


Biology, 04.02.2020 15:45




Mathematics, 04.02.2020 15:45





History, 04.02.2020 15:45

History, 04.02.2020 15:45

English, 04.02.2020 15:45

History, 04.02.2020 15:45

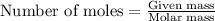 .....(1)
.....(1)