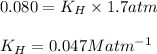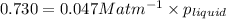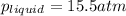Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
0.66y = 0.9x + 0.48 if y has a value of 108.45 what is the value of x?
Answers: 1

Chemistry, 22.06.2019 04:20
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1

Chemistry, 22.06.2019 06:40
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1
You know the right answer?
At a particular temperature, the solubility of He in water is 0.080 M when the partial pressure is 1...
Questions

Physics, 01.08.2019 12:00


History, 01.08.2019 12:00



Mathematics, 01.08.2019 12:10



Mathematics, 01.08.2019 12:10

Mathematics, 01.08.2019 12:10

Mathematics, 01.08.2019 12:10


Mathematics, 01.08.2019 12:10





History, 01.08.2019 12:10


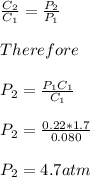
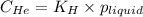
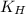 = Henry's constant =?
= Henry's constant =?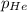 = partial pressure = 1.7 atm
= partial pressure = 1.7 atm