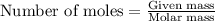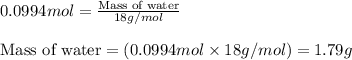Chemistry, 03.03.2020 02:52 wedestttefera
Consider the following chemical reaction: 2H2O(l)→2H2(g)+O2(g) What mass of H2O is required to form 1.3 L of O2 at a temperature of 295 K and a pressure of 0.926 atm ?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Asap! how do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 1

Chemistry, 22.06.2019 04:00
The continuous release of nuclear energy caused when one fission reaction triggered more nuclear reactions is a
Answers: 3

Chemistry, 22.06.2019 04:00
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 22.06.2019 12:00
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal.b. he is determining chemical properties that are sufficient to identify the metal.c. he is determining physical properties that are insufficient to identify the metal.d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3
You know the right answer?
Consider the following chemical reaction: 2H2O(l)→2H2(g)+O2(g) What mass of H2O is required to form...
Questions

Biology, 18.08.2020 06:01






Spanish, 18.08.2020 06:01





Mathematics, 18.08.2020 06:01


Geography, 18.08.2020 06:01




Mathematics, 18.08.2020 06:01

Mathematics, 18.08.2020 06:01

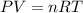
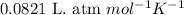
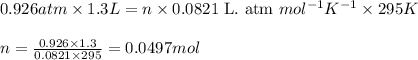
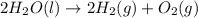
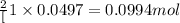 of water
of water