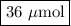Chemistry, 03.03.2020 03:26 ItzAquaZ8716
A chemist adds 240.0mL of a 1.5 x 10^-4 mol/L magnesium flouride (MgF2) solution to a reaction flask. Calculate the micromoles of magnesium fluoride the chemist has added to the flask. Be sure your answer has the correct number of significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1



Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2
You know the right answer?
A chemist adds 240.0mL of a 1.5 x 10^-4 mol/L magnesium flouride (MgF2) solution to a reaction flask...
Questions

Mathematics, 04.04.2020 05:31


Computers and Technology, 04.04.2020 05:31

Mathematics, 04.04.2020 05:31









Mathematics, 04.04.2020 05:31


Mathematics, 04.04.2020 05:31

Mathematics, 04.04.2020 05:32



Engineering, 04.04.2020 05:32

Health, 04.04.2020 05:32