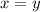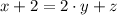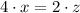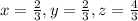Chemistry, 03.03.2020 03:33 dogsb4doods
Give the stoichiometric coefficient for oxygen when the following equation is balanced using the lowest, whole-number coefficients: ___CH4O(l) ___O2(g) -> ___CO2(g) H2O(l)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3

Chemistry, 23.06.2019 01:00
Which substance—wood or silver—is the better thermal conductor? a thermal conductor is a material that requires very little heat energy to change its temperature. explain your answer.
Answers: 3
You know the right answer?
Give the stoichiometric coefficient for oxygen when the following equation is balanced using the low...
Questions


Mathematics, 05.05.2020 06:02




Mathematics, 05.05.2020 06:03



Mathematics, 05.05.2020 06:03

Mathematics, 05.05.2020 06:03

History, 05.05.2020 06:03

Mathematics, 05.05.2020 06:03


Mathematics, 05.05.2020 06:03




Mathematics, 05.05.2020 06:03


History, 05.05.2020 06:03

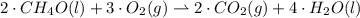
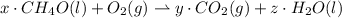
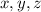 are the required variables.
are the required variables.