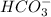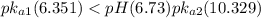Chemistry, 03.03.2020 03:40 josuemartinez1030
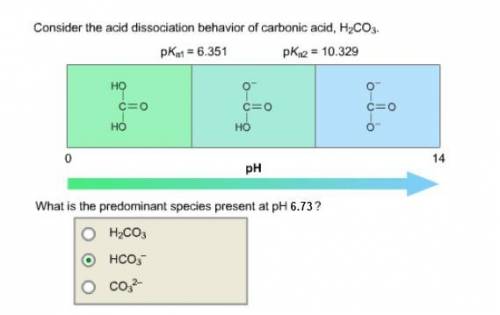
Consider the acid dissociation behavior of carbonic acid, H 2 CO 3 . A p H gradient from 0 to 14 is given. Below a p H equal to p K a 1 which is 6.351, the predominant form is H 2 C O 3. Above a p H equal to p K a 2 which is 10.329, the predominant form is C O 3 2 minus. Between the two p K a values, the predominant form is H C O 3 minus. What is the predominant species present at pH 6.73 ?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
In any energy conversion, some of the energy is lost to the environment as question 5 options: electrical energy potential energy sound energy thermal energy
Answers: 1

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2


You know the right answer?
Consider the acid dissociation behavior of carbonic acid, H 2 CO 3 . A p H gradient from 0 to 14 is...
Questions

Mathematics, 15.01.2020 22:31


Social Studies, 15.01.2020 22:31


Computers and Technology, 15.01.2020 22:31

Mathematics, 15.01.2020 22:31

History, 15.01.2020 22:31

History, 15.01.2020 22:31


Chemistry, 15.01.2020 22:31


Mathematics, 15.01.2020 22:31




History, 15.01.2020 22:31

Geography, 15.01.2020 22:31


Geography, 15.01.2020 22:31

English, 15.01.2020 22:31