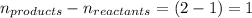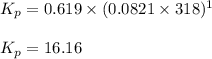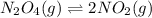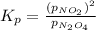Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Nebulae are enormous clouds in outer space. they are made mostly of hydrogen gas, helium gas, and dust. some nebulae glow brightly, while others do not. the stars that people see are huge, bright balls of glowing gas. they are made mostly of hydrogen and helium. which statement correctly describes other ways in which nebulae and stars are different? a. stars can form inside a nebula but a nebula can never be produced by any star. b. a star always has a higher density than a nebula. c. stars can never form inside a nebula but a nebula can be produced by any star. d. a nebula always has a higher density than a star.
Answers: 3

Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3
You know the right answer?
Part A If 50.0 gg of N2O4N2O4 is introduced into an empty 2.12 LL container, what are the partial pr...
Questions



Arts, 20.10.2020 03:01





Geography, 20.10.2020 03:01


Mathematics, 20.10.2020 03:01



Computers and Technology, 20.10.2020 03:01


Mathematics, 20.10.2020 03:01





Physics, 20.10.2020 03:01

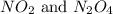 is 7.12 atm and 3.133 atm respectively.
is 7.12 atm and 3.133 atm respectively.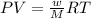
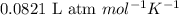
![45^oC=[45+273]K=318K](/tpl/images/0531/7110/fdbdd.png)
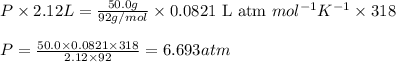
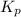 with
with 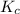 is given by the formula:
is given by the formula: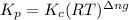
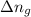 = change in number of moles of gas particles =
= change in number of moles of gas particles =