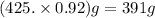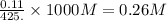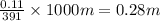A student dissolves 14.g of benzoic acid C7H6O2 in 425.mL of a solvent with a density of 0.92 g/mL. The student notices that the volume of the solvent does not change when the benzoic acid dissolves in it. Calculate the molarity and molality of the student's solution. Round both of your answers to 2 significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Which is a chemical property of iron? a. it forms iron oxide (rust) when exposed to moisture and air. b. it is a gray–black metal that is hard to the touch. c. it has a melting point of 2795°f (1536°c). d. it is a good conductor of heat
Answers: 2

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2
You know the right answer?
A student dissolves 14.g of benzoic acid C7H6O2 in 425.mL of a solvent with a density of 0.92 g/mL....
Questions



Social Studies, 03.02.2020 00:58

Business, 03.02.2020 00:58

Mathematics, 03.02.2020 00:58

English, 03.02.2020 00:58

Mathematics, 03.02.2020 00:58

English, 03.02.2020 00:58


Biology, 03.02.2020 00:58


English, 03.02.2020 00:58



Mathematics, 03.02.2020 00:58

Spanish, 03.02.2020 00:58

History, 03.02.2020 00:58

History, 03.02.2020 00:58


Health, 03.02.2020 00:58

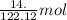 of benzoic acid = 0.11 mol of benzoic acid
of benzoic acid = 0.11 mol of benzoic acid