Chemistry, 03.03.2020 04:53 GhostElite6383
A 0.9679-g sample containing dimethylphthalate, (194.19 g/mol), and unreactive species was refluxed with 50.00 mL of 0.1215 M to hydrolyze the ester groups (this process is called saponification).
C6H4(COOCH3)2 + 2OH>> C6H4(COO)-2 + H2O
After the reaction was complete, the excess NaOH was back titrated with 32.25mL of 0.1251M HCl. Calculate the percentage of dimethylphthalate in the sample.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Naoki's bicycle has a mass of 10 kg. if naoki sits on her bicycle and starts pedaling with a force of 168 n, causing an acceleration of 2.8 m/s2, what is naoki's mass?
Answers: 1

Chemistry, 22.06.2019 07:10
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 15:30
How does a large body of water, such as the ocean, influence climate?
Answers: 1
You know the right answer?
A 0.9679-g sample containing dimethylphthalate, (194.19 g/mol), and unreactive species was refluxed...
Questions

Mathematics, 09.03.2021 17:10


Mathematics, 09.03.2021 17:10

English, 09.03.2021 17:10

History, 09.03.2021 17:10

Physics, 09.03.2021 17:10


Social Studies, 09.03.2021 17:10




Mathematics, 09.03.2021 17:10



Mathematics, 09.03.2021 17:10

Social Studies, 09.03.2021 17:10


Arts, 09.03.2021 17:10

Mathematics, 09.03.2021 17:10

Social Studies, 09.03.2021 17:10

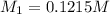
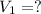

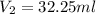
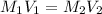
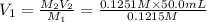
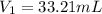
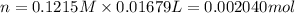

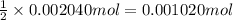 of dimethylphthalate
of dimethylphthalate