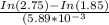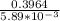Chemistry, 03.03.2020 05:45 Mariela2699
N2O5 decomposes to form NO2 and O2 with first-order kinetics. How long does it take for the N2O5 concentration to decrease from its initial value of 2.75 M to its final value of 1.85 M, if the rate constant, k, equals 5.89 × 10−3?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2

You know the right answer?
N2O5 decomposes to form NO2 and O2 with first-order kinetics. How long does it take for the N2O5 con...
Questions




Mathematics, 19.12.2020 23:20





German, 19.12.2020 23:20


Arts, 19.12.2020 23:20



Mathematics, 19.12.2020 23:20