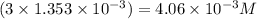Chemistry, 03.03.2020 06:10 andrew8228
A chemist prepares a solution of vanadium(III) bromide (VBr) by measuring out 0.12 g of VBr into a 300 ml. volumetric flask and filling to the mark with distilled water. Calculate the molarity of Branions in the chemist's solution. Be sure your answer is rounded to significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:40
What are the resulting coefficients when you balance the chemical equation for the combustion of ethane, c2h6? in this reaction, ethane is burned in the presence of oxygen (o2) to form carbon dioxide (co2) and water (h2o). (g)+(g)→(g)+(g)
Answers: 1


Chemistry, 22.06.2019 04:00
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1
You know the right answer?
A chemist prepares a solution of vanadium(III) bromide (VBr) by measuring out 0.12 g of VBr into a 3...
Questions

Mathematics, 27.10.2021 21:30


Geography, 27.10.2021 21:40

Mathematics, 27.10.2021 21:40

Mathematics, 27.10.2021 21:40

English, 27.10.2021 21:40


English, 27.10.2021 21:40



Mathematics, 27.10.2021 21:40


History, 27.10.2021 21:40

Physics, 27.10.2021 21:40



Chemistry, 27.10.2021 21:40



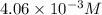
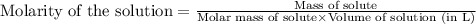
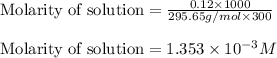
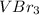
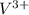 ions and 3 moles of
ions and 3 moles of 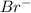 ions
ions