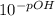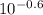A chemist must prepare 0.9 L of sodium hydroxide solution with a pH of 13.40 at 25°C. He will do this in three steps: Fill a 0.9 L volumetric flask about halfway with distilled water. Weigh out a small amount of solid sodium hydroxide and add it to the flask. Fill the flask to the mark with distilled water. Calculate the mass of sodium hydroxide that the chemist must weigh out in the second step.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:30
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3

Chemistry, 22.06.2019 20:00
State one important difference between a physical change and a chemical change?
Answers: 1

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3
You know the right answer?
A chemist must prepare 0.9 L of sodium hydroxide solution with a pH of 13.40 at 25°C. He will do thi...
Questions

Biology, 02.02.2020 14:45

Computers and Technology, 02.02.2020 14:46


Chemistry, 02.02.2020 14:46

English, 02.02.2020 14:46

Computers and Technology, 02.02.2020 15:43

Mathematics, 02.02.2020 15:43



Mathematics, 02.02.2020 15:43

Social Studies, 02.02.2020 15:43

Mathematics, 02.02.2020 15:43

English, 02.02.2020 15:43

Mathematics, 02.02.2020 15:43

Mathematics, 02.02.2020 15:43


History, 02.02.2020 15:43



Chemistry, 02.02.2020 15:43