
Write the balanced net ionic equation for the reactions that occur when the given aqueous solutions are mixed. Include the physical states. A. silver nitrate, AgNO 3 AgNO3 , and magnesium bromide, MgBr 2 MgBr2 net ionic equation: B. perchloric acid, HClO 4 HClO4 , and potassium hydroxide, KOH KOH net ionic equation: C. ammonium sulfide, ( NH 4 ) 2 S (NH4)2S , and cobalt(II) chloride, CoCl 2 CoCl2 net ionic equation:

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2


Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Chemistry, 22.06.2019 22:00
Scientists often have to deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each number in an alternate form.
Answers: 1
You know the right answer?
Write the balanced net ionic equation for the reactions that occur when the given aqueous solutions...
Questions

English, 28.11.2020 15:30

Business, 28.11.2020 15:30



Mathematics, 28.11.2020 15:30

Computers and Technology, 28.11.2020 15:30

Chemistry, 28.11.2020 15:30

Medicine, 28.11.2020 15:30

Chemistry, 28.11.2020 15:30


Social Studies, 28.11.2020 15:30

Social Studies, 28.11.2020 15:40

German, 28.11.2020 15:40


Physics, 28.11.2020 15:40

Mathematics, 28.11.2020 15:40


English, 28.11.2020 15:40

Mathematics, 28.11.2020 15:40

Mathematics, 28.11.2020 15:40

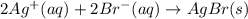
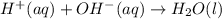
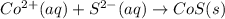
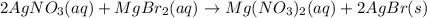
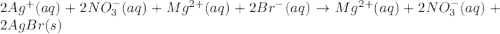
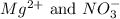 are the spectator ions.
are the spectator ions.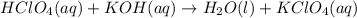
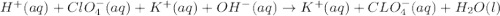
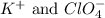 are the spectator ions.
are the spectator ions.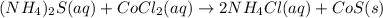
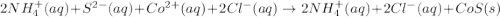
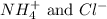 are the spectator ions.
are the spectator ions.

