Consider the second-order reaction:
2HI(g)→H2(g)+I2(g)
Use the simulation to...

Chemistry, 03.03.2020 05:56 ayoismeisalex
Consider the second-order reaction:
2HI(g)→H2(g)+I2(g)
Use the simulation to find the initial concentration [HI]0 and the rate constant k for the reaction. What will be the concentration of HI after t = 4.53×1010 s ([HI]t) for a reaction starting under the condition in the simulation?
Given from simulation:
Rate Law: k[HI]^2
k= 6.4 x 10^-9 l/(mol x s) at 500K
Initial Rate= 1.6 x 10^-7 mol/(l x s)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
After cloud droplets form, what must happen to them for precipitation to occur?
Answers: 1

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1
You know the right answer?
Questions


Mathematics, 29.05.2020 00:04




Biology, 29.05.2020 00:04


Mathematics, 29.05.2020 00:04




Computers and Technology, 29.05.2020 00:04




Mathematics, 29.05.2020 00:04



Social Studies, 29.05.2020 00:04

Biology, 29.05.2020 00:04

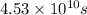 is 0.00345 mol/L.
is 0.00345 mol/L.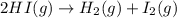
![k[HI]^2 ](/tpl/images/0531/9344/c44b5.png)
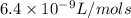
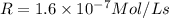
![[A_o]](/tpl/images/0531/9344/dc622.png)
![1.6\times 10^{-7} mol/L s=(6.4\times 10^{-9} L/mol s)[HI]^2](/tpl/images/0531/9344/8e7c2.png)
![[A_o]=5 mol/L](/tpl/images/0531/9344/ca62d.png)
![\frac{1}{[A]}=kt+\frac{1}{[A_o]}](/tpl/images/0531/9344/a4900.png)
![\frac{1}{[A]}=6.4\times 10^{-9} L/mol s\times 4.53\times 10^{10} s+\frac{1}{[5 mol/L]}](/tpl/images/0531/9344/3dcb7.png)
![[A]=0.00345 mol/L](/tpl/images/0531/9344/f652e.png)


