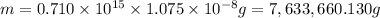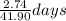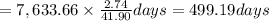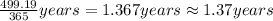Chemistry, 03.03.2020 22:34 witchhunt666
Suppose a group of volunteers is planning to build a park near a local lake. The lake is known to contain low levels of arsenic (As). Therefore, prior to starting construction, the group decides to measure the current level of arsenic in the lake. A) If a 15.7 cm3 sample of lake water is found to have 164.5 ng As, what is the concentration of arsenic in the sample in parts per billion (ppb), assuming that the density of the lake water is 1.00 g/cm3?
B) Calculate the total mass (in kg) of arsenic in the lake that the company will have to remove if the total volume of water in the lake is 0.710 km3?
C) Based on the company\'s claim and the concentration of arsenic in the lake, how many years will it take to remove all of the arsenic from the lake, assuming that there are always 365 days in a year?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

Chemistry, 22.06.2019 20:30
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1

Chemistry, 22.06.2019 21:30
How many oxygen atoms are there in 3.15 moles of hcl manganese (iv) oxide, mno2
Answers: 2
You know the right answer?
Suppose a group of volunteers is planning to build a park near a local lake. The lake is known to co...
Questions





Mathematics, 26.08.2021 09:10


Mathematics, 26.08.2021 09:10

Computers and Technology, 26.08.2021 09:10

History, 26.08.2021 09:10

History, 26.08.2021 09:10

Computers and Technology, 26.08.2021 09:10


Computers and Technology, 26.08.2021 09:10

Chemistry, 26.08.2021 09:10



Computers and Technology, 26.08.2021 09:10

History, 26.08.2021 09:10

Computers and Technology, 26.08.2021 09:10

Mathematics, 26.08.2021 09:10

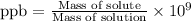
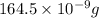
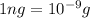
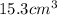
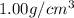
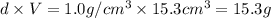
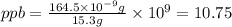
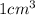 of lake water =
of lake water = 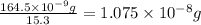
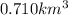 lake water be m.
lake water be m.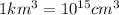
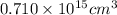 lake water :
lake water :