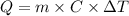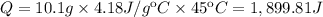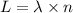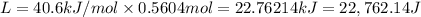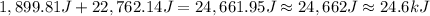Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2
You know the right answer?
Calculate the energy (in kJ) required to heat 10.1 g of liquid water from 55 oC to 100 oC and change...
Questions

Mathematics, 24.02.2021 01:40



Mathematics, 24.02.2021 01:40




Mathematics, 24.02.2021 01:40

Mathematics, 24.02.2021 01:40



Mathematics, 24.02.2021 01:40

History, 24.02.2021 01:40




Mathematics, 24.02.2021 01:40


Biology, 24.02.2021 01:40