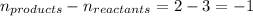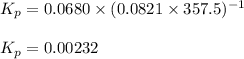Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1


Chemistry, 22.06.2019 15:30
Which of the following are correct values for the ideal gas laws constant r
Answers: 1

Chemistry, 22.06.2019 16:50
Ajet plane is speeding down the runway during takeoff. air resistance is not negligible. identify the forces on the jet.
Answers: 3
You know the right answer?
2 SO2(g) + O2(g) 2 SO3(g) Assume that Kc = 0.0680 for the gas phase reaction above. Calculate the co...
Questions



Mathematics, 23.04.2020 18:51





History, 23.04.2020 18:52


History, 23.04.2020 18:52



Spanish, 23.04.2020 18:52

Business, 23.04.2020 18:52

Health, 23.04.2020 18:52





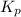 for this reaction at 84.5°C is 0.00232
for this reaction at 84.5°C is 0.00232 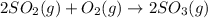
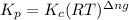
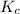 = equilibrium constant in terms of concentration = 0.0680
= equilibrium constant in terms of concentration = 0.0680 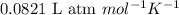
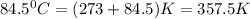
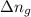 = change in number of moles of gas particles =
= change in number of moles of gas particles =