
Chemistry, 04.03.2020 23:43 angel213326
Magnesium (used in the manufacture of light alloys) reacts with iron(III) chloride to form magnesium chloride and iron. A mixture of 41.0 g of magnesium and 175.0 g of iron(III) chloride is allowed to react. Identify the limiting reactant and determine the mass of the excess reactant present in the vessel when the reaction is complete.

Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2

Chemistry, 22.06.2019 21:00
In the experiment you asked to react hydrochloric acid and with sodium hydroxide. when measuring the volume of the reactants, which instrument would give the greatest precision.
Answers: 3
You know the right answer?
Magnesium (used in the manufacture of light alloys) reacts with iron(III) chloride to form magnesium...
Questions

History, 28.09.2019 23:50

Mathematics, 28.09.2019 23:50

English, 28.09.2019 23:50

Business, 28.09.2019 23:50

Mathematics, 28.09.2019 23:50

Mathematics, 28.09.2019 23:50

Physics, 28.09.2019 23:50

Chemistry, 28.09.2019 23:50


Advanced Placement (AP), 28.09.2019 23:50

English, 28.09.2019 23:50




Social Studies, 28.09.2019 23:50





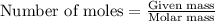 .....(1)
.....(1)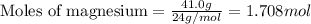
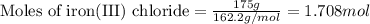
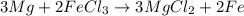
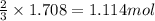 of iron(III) chloride
of iron(III) chloride



