
Chemistry, 04.03.2020 23:26 TheOneandOnly003
A compound is 92.2% Carbon and 7.76% Hydrogen. The formula mass of the compound is 78.1 g. Calculate the empirical formula and molecular formula of the compound.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Different isotopes indicate that an element will have different numbers of
Answers: 2

Chemistry, 22.06.2019 14:00
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1


You know the right answer?
A compound is 92.2% Carbon and 7.76% Hydrogen. The formula mass of the compound is 78.1 g. Calculate...
Questions


Mathematics, 29.10.2020 02:00

Chemistry, 29.10.2020 02:00

Social Studies, 29.10.2020 02:00

Mathematics, 29.10.2020 02:00

Geography, 29.10.2020 02:00





Mathematics, 29.10.2020 02:00


Computers and Technology, 29.10.2020 02:00


Mathematics, 29.10.2020 02:00


Mathematics, 29.10.2020 02:00

Advanced Placement (AP), 29.10.2020 02:00

Advanced Placement (AP), 29.10.2020 02:00

Mathematics, 29.10.2020 02:00

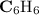
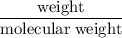
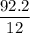
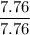
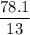
 .
.

