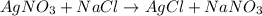Chemistry, 05.03.2020 00:22 tytybruce2
On a balance, you have beakers of AgNO3AgNO3 solution and NaClNaCl solution. When mixed, they will form AgCl(s)AgCl(s). What will happen to the mass of the new mixture?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no2]2 ? second order 3/2 order third order zero order none of the listed answers are correct
Answers: 3

Chemistry, 23.06.2019 11:20
Try to reduce the amount of leftover ingredients by changing the amount of one, two, or all three starting ingredients. show your stoichiometric calculations below. water 946.36 g sugar 196.86 g lemon juice193.37 g
Answers: 2

Chemistry, 23.06.2019 13:30
How many more valence electrons does sodium need to have a full outer valence shell
Answers: 1

Chemistry, 23.06.2019 14:30
2.38g of black copper (ii) oxide is completely reduced by hydrogen to give copper and water. what are the masses of copper and water formed? ?
Answers: 1
You know the right answer?
On a balance, you have beakers of AgNO3AgNO3 solution and NaClNaCl solution. When mixed, they will f...
Questions


Mathematics, 11.08.2021 01:00


Mathematics, 11.08.2021 01:00

Mathematics, 11.08.2021 01:00


Social Studies, 11.08.2021 01:00


English, 11.08.2021 01:00



Mathematics, 11.08.2021 01:00

Social Studies, 11.08.2021 01:00

Mathematics, 11.08.2021 01:00

Biology, 11.08.2021 01:00


Mathematics, 11.08.2021 01:00

Mathematics, 11.08.2021 01:00


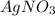 is added to NaCl then the compound formed will have same mass as that of reactants.
is added to NaCl then the compound formed will have same mass as that of reactants.