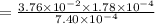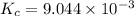Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1


Chemistry, 22.06.2019 14:30
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1

Chemistry, 22.06.2019 17:30
What type of organic molecule comprises the majority of a potato?
Answers: 1
You know the right answer?
Some COCl2 is placed in a sealed flask and heated to 756 K. When equilibrium is reached, the flask i...
Questions


Mathematics, 09.11.2019 13:31

Mathematics, 09.11.2019 13:31


Business, 09.11.2019 13:31


Mathematics, 09.11.2019 13:31


English, 09.11.2019 13:31


Mathematics, 09.11.2019 13:31

Social Studies, 09.11.2019 13:31



Mathematics, 09.11.2019 13:31

English, 09.11.2019 13:31


Physics, 09.11.2019 13:31


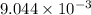 is the value of the equilibrium constant for this reaction at 756 K.
is the value of the equilibrium constant for this reaction at 756 K.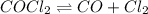
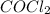
![[COCl_2]=7.40\times 10^{-4} M](/tpl/images/0534/1166/1134d.png)
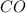
![[CO]=3.76\times 10^{-2} M](/tpl/images/0534/1166/484f5.png)
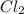
![[Cl_2]=1.78\times 10^{-4} M](/tpl/images/0534/1166/d4643.png)
![K_c=\frac{[CO][Cl_2]}{[COCl_2]}](/tpl/images/0534/1166/59c52.png)