
Chemistry, 05.03.2020 05:16 calmicaela12s
Consider the unbalanced equation for the combustion of hexane: C6H14 (g) O2 (g) --> CO2 (g) H2O (g) Balance the equation and determine how many moles of O2 are required to react completely with 7.2 moles of C6H14.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 07:40
The formation of a solid, also known as a is an indication of a chemical change. precipitate particulate particle powder
Answers: 3

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

You know the right answer?
Consider the unbalanced equation for the combustion of hexane: C6H14 (g) O2 (g) --> CO2 (g) H2O (...
Questions


Mathematics, 09.06.2020 18:57

Mathematics, 09.06.2020 18:57



Mathematics, 09.06.2020 18:57





Mathematics, 09.06.2020 18:57




Social Studies, 09.06.2020 18:57

Advanced Placement (AP), 09.06.2020 18:57

English, 09.06.2020 18:57

English, 09.06.2020 18:57


Mathematics, 09.06.2020 18:57

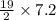 .
.

