Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:50
An aqueous solution of potassium hydroxide is standardized by titration with a 0.194 m solution of hydrobromic acid. if 25.2 ml of base are required to neutralize 24.2 ml of the acid, what is the molarity of the potassium hydroxide solution? m potassium hydroxide
Answers: 2

Chemistry, 23.06.2019 00:30
Which of the following best describes technology a. something created for only scientists to use b.the method of thinking that scientists use. c.the application of engineering to create useful products. c. a scientific idea
Answers: 1

Chemistry, 23.06.2019 03:20
High-pressure liquid chromatography (hplc) is a method used in chemistry and biochemistry to purify chemical substances. the pressures used in this procedure range from around 500 kilopascals (500,000 pa) to about 60,000 kpa (60,000,000 pa). it is often convenient to know the pressure in torr. if an hplc procedure is running at a pressure of 1.03×108 pa , what is its running pressure in torr?
Answers: 3

Chemistry, 23.06.2019 14:50
Write an equation to show action of positive and negative catalyst
Answers: 1
You know the right answer?
A solution is prepared by dissolving 27.0 g of urea [(NH2)2CO], in 150.0 g of water. Calculate the b...
Questions

Mathematics, 21.06.2019 19:00


Biology, 21.06.2019 19:00









History, 21.06.2019 19:00




Mathematics, 21.06.2019 19:00



Mathematics, 21.06.2019 19:00

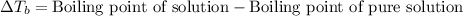
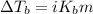
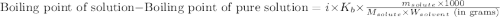
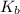 = molal boiling point elevation constant = 0.52°C/m.g
= molal boiling point elevation constant = 0.52°C/m.g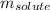 = Given mass of solute (urea) = 27.0 g
= Given mass of solute (urea) = 27.0 g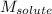 = Molar mass of solute (urea) = 60 g/mol
= Molar mass of solute (urea) = 60 g/mol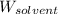 = Mass of solvent (water) = 150.0 g
= Mass of solvent (water) = 150.0 g