
Chemistry, 05.03.2020 10:11 trevorhenyan51
A certain mass of carbon reacts with 13.6 g of oxygen to form carbon monoxide. grams of oxygen would react with that same mass of carbon to form carbon dioxide, according to the law of multiple proportions.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:00
The number to the right of an element's symbol (ex. c-12) identifies the of an isotope.
Answers: 1

Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1

Chemistry, 22.06.2019 21:30
If you burn 46.6 g of hydrogen and produce 416 g of water, how much oxygen reacted
Answers: 3
You know the right answer?
A certain mass of carbon reacts with 13.6 g of oxygen to form carbon monoxide. grams of oxygen woul...
Questions




Mathematics, 27.11.2019 03:31



Mathematics, 27.11.2019 03:31

Mathematics, 27.11.2019 03:31

History, 27.11.2019 03:31











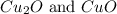
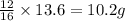 of carbon
of carbon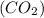 :
: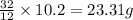 of oxygen
of oxygen

