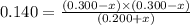Consider mixture C, which will cause the net reaction to proceed in reverse. Concentration (M)initial:change:equilibrium:[XY]0 .200+x0.200+x←net⇌[X]0.300−x0.300−x +[Y]0.300−x0.300−x The change in concentration, x, is positive for the reactants because they are produced and negative for the products because they are consumed.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:10
Nitrogen (n), phosphorus (p), and potassium (k) are the main nutrients in plant fertilizers. according to an industry convention, the numbers on the label refer to the mass percents of n, p2o5, and k2o, in that order. calculate the n: p: k ratio of a 30: 10: 10 fertilizer in terms of moles of each element, and express it as x: y: 1.0.
Answers: 1

Chemistry, 22.06.2019 12:00
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2
You know the right answer?
Consider mixture C, which will cause the net reaction to proceed in reverse. Concentration (M)initia...
Questions


Mathematics, 17.12.2020 21:00


Computers and Technology, 17.12.2020 21:00



Social Studies, 17.12.2020 21:00

Mathematics, 17.12.2020 21:00

Chemistry, 17.12.2020 21:00


Mathematics, 17.12.2020 21:00


Social Studies, 17.12.2020 21:00


English, 17.12.2020 21:00




Mathematics, 17.12.2020 21:00

Mathematics, 17.12.2020 21:00

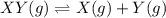
![K_c=\farc{[X][Y]}{[XY]}](/tpl/images/0534/4698/91cd9.png)