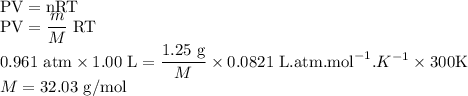Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Check the correct box to describe the periodic trends in electronegativity. electronegativity across a period: decreases. increases. electronegativity down a group: decreases. increases.
Answers: 2

Chemistry, 22.06.2019 02:00
For each of the following types of reactions, write a general reaction formula in the symbolic form—for example, a + b → ab. single-displacement double-displacement synthesis decomposition
Answers: 1

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 08:30
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1
You know the right answer?
What is the molar mass of a 1.25 g sample of gas that occupies a volume of 1.00L at a pressure of 0....
Questions


Physics, 03.11.2020 05:10

History, 03.11.2020 05:10

Mathematics, 03.11.2020 05:10


Mathematics, 03.11.2020 05:10




Mathematics, 03.11.2020 05:10

Mathematics, 03.11.2020 05:10

Mathematics, 03.11.2020 05:10


Mathematics, 03.11.2020 05:10



Mathematics, 03.11.2020 05:10

English, 03.11.2020 05:10

Medicine, 03.11.2020 05:10

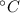 = 300 K
= 300 K