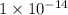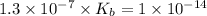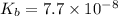Chemistry, 22.12.2019 15:31 superfly903
The ka of an acid is 1.3 x 10^–7. based on the ka and its relationship with kw, what is the value of kb? use kakb=kw
1.3 x 10^–21
7.7 x 10^–15
1.3 x 10^–14
7.7 x 10^–8

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2

Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3

You know the right answer?
The ka of an acid is 1.3 x 10^–7. based on the ka and its relationship with kw, what is the value of...
Questions

Mathematics, 12.03.2020 18:57


Geography, 12.03.2020 18:57

Mathematics, 12.03.2020 18:57



English, 12.03.2020 18:57

Mathematics, 12.03.2020 18:57




Social Studies, 12.03.2020 18:57


Computers and Technology, 12.03.2020 18:57





Mathematics, 12.03.2020 18:57

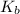 is,
is, 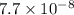
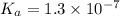
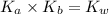
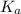 = dissociation constant of an acid =
= dissociation constant of an acid = 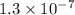
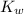 = dissociation constant of water =
= dissociation constant of water =