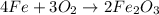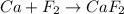Chemistry, 29.08.2019 04:30 thompsonhomes1
Classify the following reactions as either redox, acid-base, or precipitation. a) (cl2) + (2oh-) --> (cl-) + (clo-) + (h2o). b) already got. c) (nh3) + (h+) --> (nh4+). d) (4fe) + (3o2) --> (2fe2o3). e) (ca) + (f2) --> (caf2)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:20
Why does the sun appear to be the brightest star in the sky? a- its apparent brightness is much greater than other stars. b- it burns more gas, making it brighter than any other star. c- it is the largest star in the galaxy, so it is the brightest star. d- its relative distance to earth is closer than the other stars.
Answers: 1

Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2

Chemistry, 22.06.2019 19:50
Which sentence from holes contains an implied personality trait? stanley and his parents had tried to pretend that he was just going away to camp for a while, just like rich kids do. he'd just been in the wrong place at the wrong time. stanley felt somewhat dazed as the guard unlocked his handcuffs and led him off the bus. stanley nodded to show he understood
Answers: 3

Chemistry, 23.06.2019 05:00
Match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) amount of product predicted to be produced by the given reactants theoretical yield c) reactant that can produce more of the product
Answers: 3
You know the right answer?
Classify the following reactions as either redox, acid-base, or precipitation. a) (cl2) + (2oh-) --&...
Questions






History, 05.06.2020 19:03


Mathematics, 05.06.2020 19:03

Mathematics, 05.06.2020 19:03




Mathematics, 05.06.2020 19:03

Social Studies, 05.06.2020 19:03






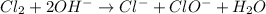
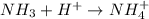
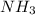 is a base and
is a base and 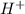 is an acid react to give
is an acid react to give 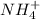 as a salt.
as a salt.