
Chemistry, 27.09.2019 11:30 Raymond123
1. in this experiment, what property of nacl is used to separate it from the other two components? is this a chemical or physical property? nacl is the only compound in this mixture that is soluble in water, so its solubility in water is the property used to separate it. this is a physical property, because you are doing nothing to change the actual chemical composition. it's just solvated by water. 2. in this experiment, what property of caco3 is used to separate it from sio2? is this a chemical or physical property? the property of caco3 used to seperate it from sio2 is its reactivity with acetic acid. by adding the acetic acid, you develop carbon dioxide (which bubbles out of solution), and cacl2, which is soluble in water. you are undergoing a chemical reaction, so this is a chemical property. 3. an unknown sample of a mixture of salt and sand weighs 7.52 g before washing with water and 3.45 g after. what is the percent of sand in the sample? (show all work.) 7.52g salt and sand, 3.45g sand, 4.07g salt (the difference in before and after water shows how much salt you had) 3.45 g sand / 7.52g total * 100 = 45.9% sand to check that this is correct, find the percentage of salt as well. 4.07g salt / 7.52g * 100 = 54.1% salt 45.9% + 54.1% = 100%. everything is accounted for, so this must be the composition of the mixture.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2


Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1

Chemistry, 22.06.2019 19:00
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2
You know the right answer?
1. in this experiment, what property of nacl is used to separate it from the other two components?...
Questions


History, 05.07.2019 12:20




History, 05.07.2019 12:20

Mathematics, 05.07.2019 12:20

History, 05.07.2019 12:20






Chemistry, 05.07.2019 12:20


Physics, 05.07.2019 12:20




Mathematics, 05.07.2019 12:20

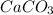 first separation was done by dissolving the mixture in water. In the first step the NaCl will get dissolved whereas,
first separation was done by dissolving the mixture in water. In the first step the NaCl will get dissolved whereas, 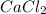 which gets dissolved into the solution of HCl. Here, first decantation occurs and then extraction is done. Second, extraction is done using potassium carbonate in it which separates
which gets dissolved into the solution of HCl. Here, first decantation occurs and then extraction is done. Second, extraction is done using potassium carbonate in it which separates 

