
Chemistry, 06.03.2020 23:26 lilquongohard
You carefully weigh out 16.00 g of CaCO3 powder and add it to 64.80 g of HCl solution. You notice bubbles as a reaction takes place. You then weigh the resulting solution and find that it has a mass of 74.24 g . The relevant equation is CaCO_3(s) + 2HCl(aq) rightarrow H_2O(l) + CO_2(g) + CaCl_2(aq) Assuming no other reactions take place, what mass of CO_2 was produced in this reaction?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Flourine is found to undergo 10% radioactivity decay in 366 minutes determine its halflife
Answers: 3

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1

Chemistry, 22.06.2019 20:00
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3
You know the right answer?
You carefully weigh out 16.00 g of CaCO3 powder and add it to 64.80 g of HCl solution. You notice bu...
Questions



Mathematics, 14.01.2021 20:40

Mathematics, 14.01.2021 20:40

Physics, 14.01.2021 20:40

Computers and Technology, 14.01.2021 20:40

Chemistry, 14.01.2021 20:40



Mathematics, 14.01.2021 20:40


Mathematics, 14.01.2021 20:40

History, 14.01.2021 20:40



English, 14.01.2021 20:40

English, 14.01.2021 20:40

Mathematics, 14.01.2021 20:40


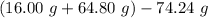
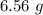
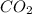 produced in this reaction was 6.56 grams
produced in this reaction was 6.56 grams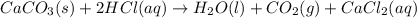
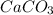 + mass of
+ mass of 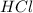 = 16.00 + 64.80 = 80.80 g
= 16.00 + 64.80 = 80.80 g

