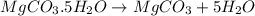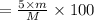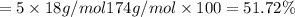You are in space and running out of water. You do have a great deal of magnesium carbonate pentahydrate. It is possible to extract the water from this. Determine the percent of water by mass in the hydrate magnesium carbonate pentahydrate (MgCO_3 middot 5H_2O). If your answer is 17%, enter 17, do not put in the percent sign. As an introduction to his class, Severus Snape teaches the first years at Hogwarts about hydrates. He begins with equations representing various dehydrations. Which of the following equations properly represents the dehydration of magnesium carbonate pentahydrate (MgCO_3 middot 5H_2O)?
MgCO_3 middot H_2O MgCO_3 + H_2O MgCO_3 middot 5H_2O
MgCO_3 + 5H_2O MgCO_3 middot 5H_2O MgCO_3 + H_2O
MgCO_3 middot 5H_2O MgCO_3 + 5H_2O

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3


Chemistry, 22.06.2019 18:40
What is one real world example of a colligative property?
Answers: 2

Chemistry, 23.06.2019 03:20
High-pressure liquid chromatography (hplc) is a method used in chemistry and biochemistry to purify chemical substances. the pressures used in this procedure range from around 500 kilopascals (500,000 pa) to about 60,000 kpa (60,000,000 pa). it is often convenient to know the pressure in torr. if an hplc procedure is running at a pressure of 1.03×108 pa , what is its running pressure in torr?
Answers: 3
You know the right answer?
You are in space and running out of water. You do have a great deal of magnesium carbonate pentahydr...
Questions




Mathematics, 01.02.2020 00:01


History, 01.02.2020 00:01




Mathematics, 01.02.2020 00:01



Computers and Technology, 01.02.2020 00:01


Health, 01.02.2020 00:01


History, 01.02.2020 00:01


English, 01.02.2020 00:01