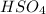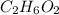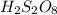Chemistry, 06.03.2020 23:27 SoccerHalo
What are the empirical formulas of a) ethylene glycol, a radiator antifreeze, molecular formula C2H6O2 b) peroxodisulfuric acid, a compound used in bleaching agents, molecular formula H2S2O8

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 21:00
Which property of water causes water drops to bead on a freshly waxed car?
Answers: 2

Chemistry, 23.06.2019 02:50
Dumbledore decides to gives a surprise demonstration. he starts with a hydrate of na2co3 which has a mass of 4.31 g before heating. after he heats it he finds the mass of the anhydrous compound is found to be 3.22 g. he asks everyone in class to determine the integer x in the hydrate: na2co3·xh2o; you should do this also. round your answer to the nearest integ
Answers: 2
You know the right answer?
What are the empirical formulas of a) ethylene glycol, a radiator antifreeze, molecular formula C2H6...
Questions

Biology, 24.03.2021 09:40



Mathematics, 24.03.2021 09:40


Spanish, 24.03.2021 09:40

Mathematics, 24.03.2021 09:40

English, 24.03.2021 09:40


Spanish, 24.03.2021 09:40


History, 24.03.2021 09:40

Mathematics, 24.03.2021 09:40

Mathematics, 24.03.2021 09:40






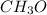 and
and