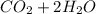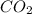Go to the Molecules mode by selecting the icon at the bottom. Notice that there are three real reactions: the synthesis of water, the synthesis of ammonia, and the combustion of methane. 1)Select each of the reactions and observe the reactants taking part in the reactions. 2)Suppose you are carrying out each of these reactions starting with five moles of each reactant, observe that some reactants are in excess whereas some reactants limit the amount of products formed. 3)Classify each of the reactants as a limiting reactant or an excess reactant for a reaction starting with five moles of each reactant. 4)Drag the appropriate items to their respective bins. View Available Hint(s) ResetHelp.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 16:00
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2

Chemistry, 23.06.2019 00:30
How can you write e method for the experiment of separating sand from water by filtration process? 1-materials 2-steps 3-conclusion also the same for the separating process of water and salt by filtration or distillation. quick because i need to finish my hw
Answers: 2
You know the right answer?
Go to the Molecules mode by selecting the icon at the bottom. Notice that there are three real react...
Questions

Mathematics, 13.09.2019 20:10












Engineering, 13.09.2019 20:10






Medicine, 13.09.2019 20:10

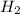 is limiting reactant ;
is limiting reactant ; 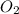 is excess reactant
is excess reactant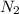 is excess reactant
is excess reactant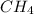 is excess reactant
is excess reactant 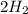 +
+ 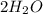
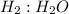
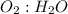
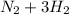 →
→ 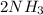
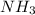
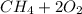 →
→