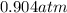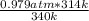Chemistry, 06.03.2020 23:49 RoyalGurl01
A sealed container holding 0.0255 L of an ideal gas at 0.979 atm and 67 ∘ C is placed into a refrigerator and cooled to 41 ∘ C with no change in volume. Calculate the final pressure of the gas. P = atm

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:40
If equal masses of the listed metals were collected , which would have a greatest volume ? a. aluminum 2.70,b.zinc7.14,c.copper 8.92,d.lead 11.34
Answers: 2

Chemistry, 22.06.2019 13:00
Adepositional also feature that forms where a stream enters a lake or an ocean is a
Answers: 2

Chemistry, 22.06.2019 17:30
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3
You know the right answer?
A sealed container holding 0.0255 L of an ideal gas at 0.979 atm and 67 ∘ C is placed into a refrige...
Questions

Mathematics, 20.09.2020 06:01




Computers and Technology, 20.09.2020 06:01

Mathematics, 20.09.2020 06:01



History, 20.09.2020 06:01






History, 20.09.2020 06:01

History, 20.09.2020 06:01

History, 20.09.2020 06:01

Health, 20.09.2020 06:01

Social Studies, 20.09.2020 06:01